Salt Water Is an Example of a Strong Electrolyte Solution
Sugar for example dissolves readily in water but remains in the water as molecules not as ions. Sodium is the electrolyte responsible for controlling the total amount of water in your body.

Electrolytes Definition Overview Expii
What is a weak electrolyte example.
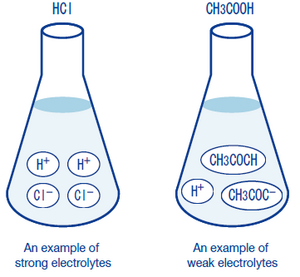
. These chemicals completely dissociate into ions in aqueous solution. Strong electrolytes include the strong acids strong bases and salts. Is the salt water solution hypertonic or hypotonic to the cucumber.
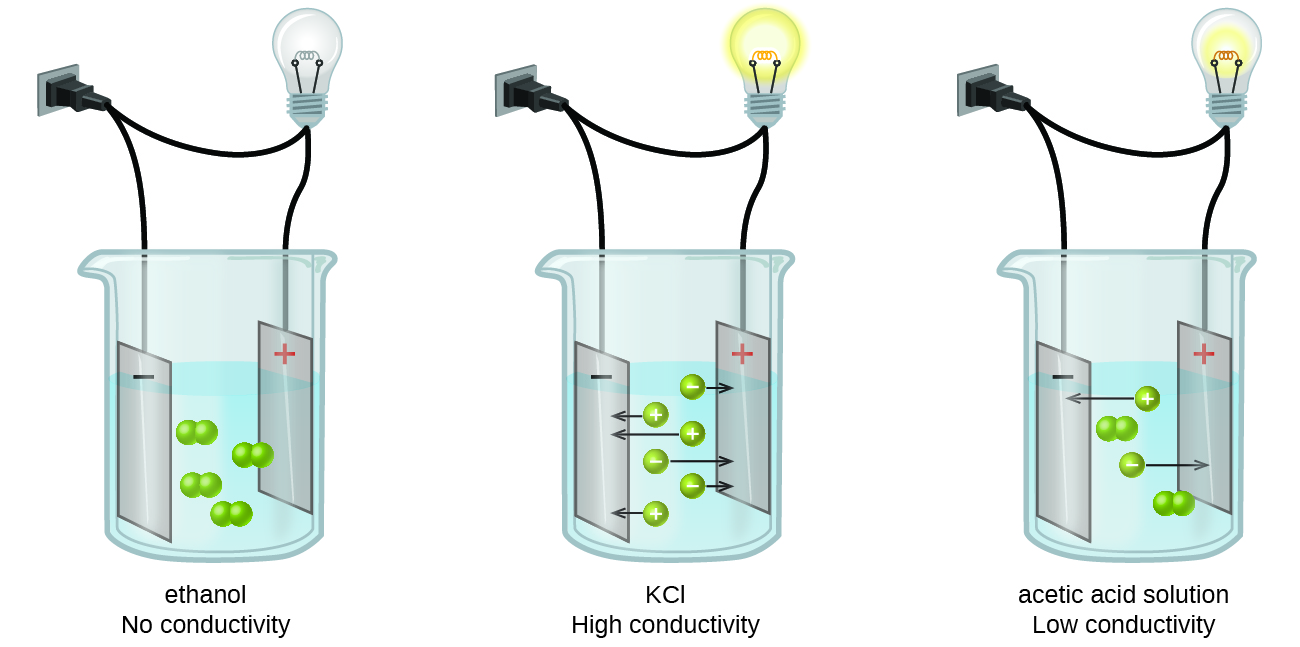
OHandHCN are weak electrolytes as they dont undergo complete dissociation whereas CH 3. Electrolyte solutions are normally formed when a salt is placed into a solvent such as water. Aqueous salt solution is a strong electrolyte.
NaCls Na aq Cl aq. A chemical equation for a strong electrolyte will contain a one-way arrow and the products will be ions. KClO3 is a salt and breaks up completely in an aqueous solution therefore it is a strong electrolyte.
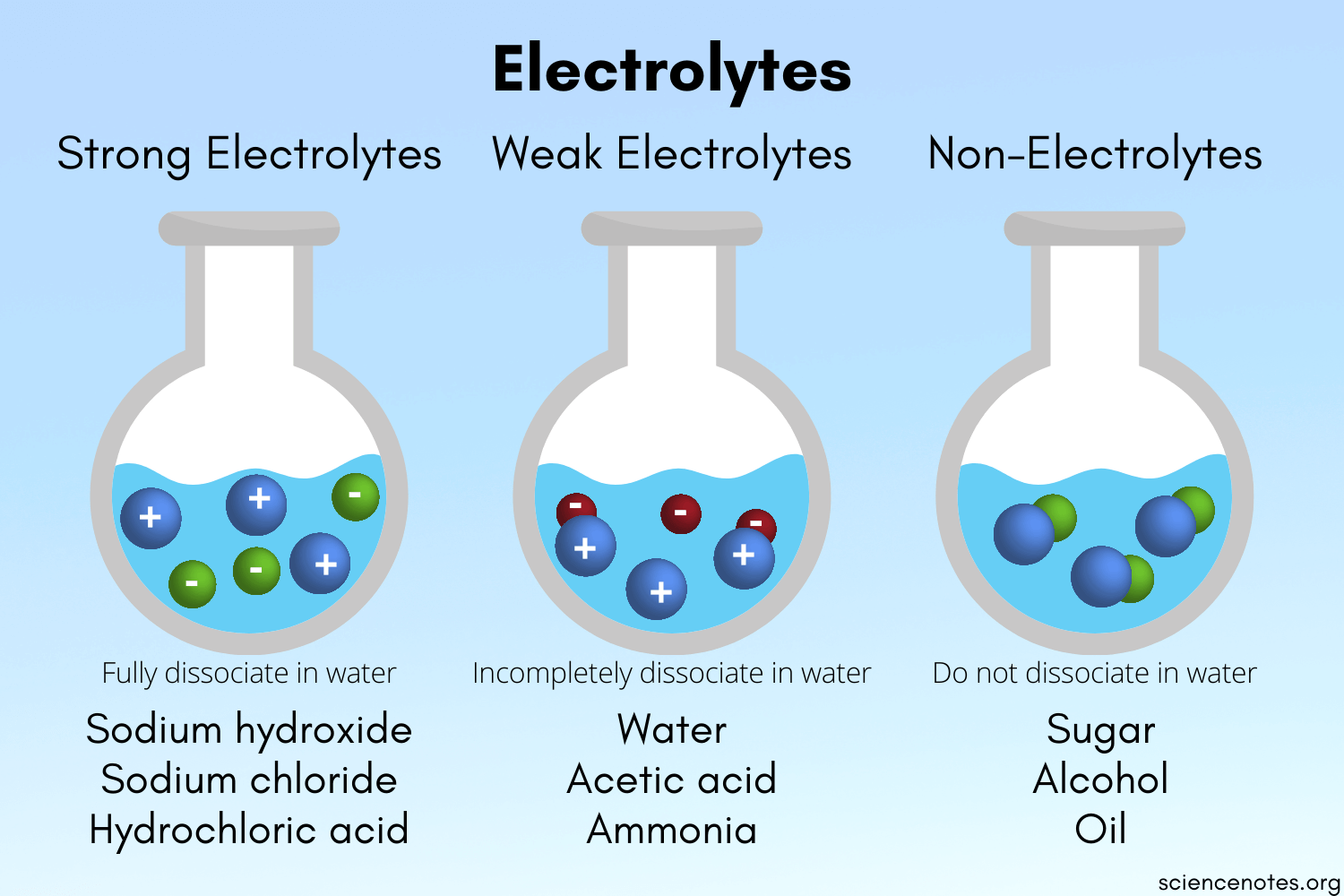
There are two types of electrolyte solutions which are strong and weak electrolytes. The difference is in the number of ions formed when the solute is dissolved. Strong acids strong bases and ionic salts that are not weak acids or bases are strong electrolytes.
Why is HF in water considered a weakly acidic solution while HCl and HBr in water are considered strongly acidic solutions. Strong electrolytes are substances that completely break apart into ions when dissolved. Sugar is classified as a non-electrolyte.
On the other hand those compounds which produce a small number of ions in solution are weak electrolytes. Solutions in which electric currents cannot run through are said to be. Table salt also known as Sodium Chloride NaCl is the most common example of the electrolyte.
The most familiar example of a strong electrolyte is table salt sodium chloride. KCl because it is highly soluble would be a strong electrolyte. For example when table salt NaCl is placed in water the salt a solid dissolves into its component ions according to the dissociation reaction.
CH3CO2H has four acidic hydrogens. Weak Electrolyte Examples HC 2 H 3 O 2 acetic acid H 2 CO 3 carbonic acid NH 3 ammonia and H 3 PO 4 phosphoric acid are all examples of weak electrolytes. In aqueous solution the NaCl crystals dissociate in their entirety to produce Na and Cl ions which end up surrounding themselves with water molecules.
Strong electrolytes will easily produce ions when dissolved. The most familiar example of a strong electrolyte is table salt sodium chloride. An electrolyte imbalance whether too much or.
For example a salt like KCl would produce an electrolyte solution. The compounds which undergo complete dissociation when dissolved in water are regarded as string electrolytes. An ionic compound for example sodium chloride dissolved in water is called an electrolyte because it conducts electricity.
COONa and NaOH being ionic salt and Strong Base respectively undergo complete dissociation. NaCl s Na aq Cl- aq. SrOH 2 - strontium hydroxide.
Is sodium chloride a nonelectrolyte. It is an ionic compound that dissociates into sodium and chloride ions when dissolved in water according to the dissociation reaction. The most representative example of an electrolyte is common salt sodium chloride NaCl.
Ionic compound dissolved in water. Examples of electrolyte solutions are acid base and salt solutions. Those compounds which produce a large number of ions in solution are called strong electrolytes.
It is then said that a salt behaves like a strong electrolyte when its solubility is very high in water. HBr - hydrobromic acid. HCl - hydrochloric acid.
Strong electrolytes are substances that completely break apart into ions when dissolved. Soluble ionic compounds are strong electrolytesOne example is potassium fluoride KF dissolved in waterStrong acids and bases are also strong electrolytes. A cucumber placed in a briny salt water solution makes a pickle.
A HF does not dissolve in water B HF has a weak conjugate base F-. Sugar for example dissolves readily in water but remains in the water as molecules not as ions. HCl hydrochloric acid H 2 SO 4 sulfuric acid NaOH sodium hydroxide and KOH potassium hydroxide are all strong electrolytes.
A strong electrolyte will completely dissociate break apart into ions in solution and will cause a strong or bright light. This balance is critically important for things like hydration nerve impulses muscle function and pH level. Exert osmotic pressure keeping body fluids in their own compartments.
NaOH - sodium hydroxide. No - NaCl is a strong electrolyte but only in water solution or after melting. Salts much have high solubility in the solvent to act as strong electrolytes.
NaCl - sodium chloride. Solid gas liquid. Electrolytes are important body constituents because they Conduct electricity essential for muscle and nerve function.
HI - hydroiodic acid. Sugar is classified as a non-electrolyte.
Belum ada Komentar untuk "Salt Water Is an Example of a Strong Electrolyte Solution"
Posting Komentar